Communications
Digital Dose: Lead Generation Ads and Influencer Faux Pas – What You Need to Know

Welcome to your dose of trending news from the digital universe, courtesy of the Spectrum Science Innovation Team.
In this edition of Digital Dose, we’re diving into Reddit’s new ad format and the FDA’s flag on pharmaceutical company Kaléo’s sponsored Instagram post with Brittany Mahomes.
Reddit Launches Lead Generation Ads
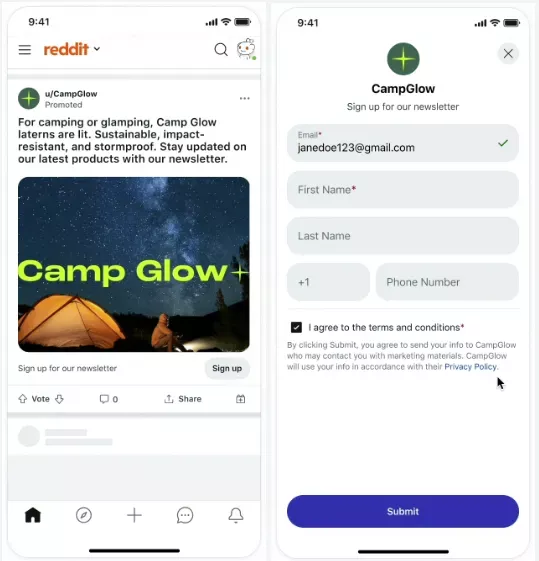
Reddit marketers can now gather prospective customer info directly from their promotions in the Reddit app with recently launched lead generation ads, now in global public beta testing. Users interested in learning more can opt into sharing their information, such as name and email addresses in a mobile-optimized design without leaving the platform, making for more seamless data collection. Read More.
Why it matters: Lead generation ads can be a valuable way to further engage interested users, particularly valuable for those who are able to feed information directly from their ads into a CRM. The ability to target specific Reddit interest-based communities may enable advertisers to reach users they are not otherwise able to target on traditional social channels like Facebook or X.
FDA Calls Foul Over Brittany Mahomes’ Kaléo Instagram Post
The FDA sent pharmaceutical company Kaléo an untitled letter asking them to change the promotion of its epinephrine injection product, Auvi-Q. The letter expresses concern that Brittany Mahomes, wife of NFL quarterback Patrick Mahomes, published an Instagram post sponsored by Auvi-Q and promoted use of the product without any reference to the risks associated with its use (the post has since been taken down). Read More.
Why it matters: The FDA’s Office of Prescription Drug Promotion uses untitled letters sparingly (only two have been issued to date this year) to call attention to false or misleading information. We use them to guide our work on behalf of our clients to ensure our communications remain compliant with FDA guidelines.
While Mahomes’ post included direction to visit the Auvi-Q Instagram account “for important safety information,” that was not enough for the FDA. Any content that covers the benefits of a drug needs to include risk information and—as this example shows—linking to or sharing where those details can be found does not cover that requirement.
Interested in more? Don’t miss these additional digital headlines:
- X Adds New Setting to Opt Out of Data Sharing with Grok, The X AI Search Assistant [Windows Central]
Have questions or comments on the content of this blog? Email Innovation@SpectrumScience.com
Perspectives

Communications
J.P. Morgan Healthcare Conference 2024: Unity and Hope Amid Industry Challenges

Communications
Attending HLTH 2024: Where Networking, Brand Boosting and Cool Collide

Communications
A Reflection on Mentoring Future Biotech Communicators

Communications
Top 5 Ways Consumer Brands Make an Impact at Medical Meetings





